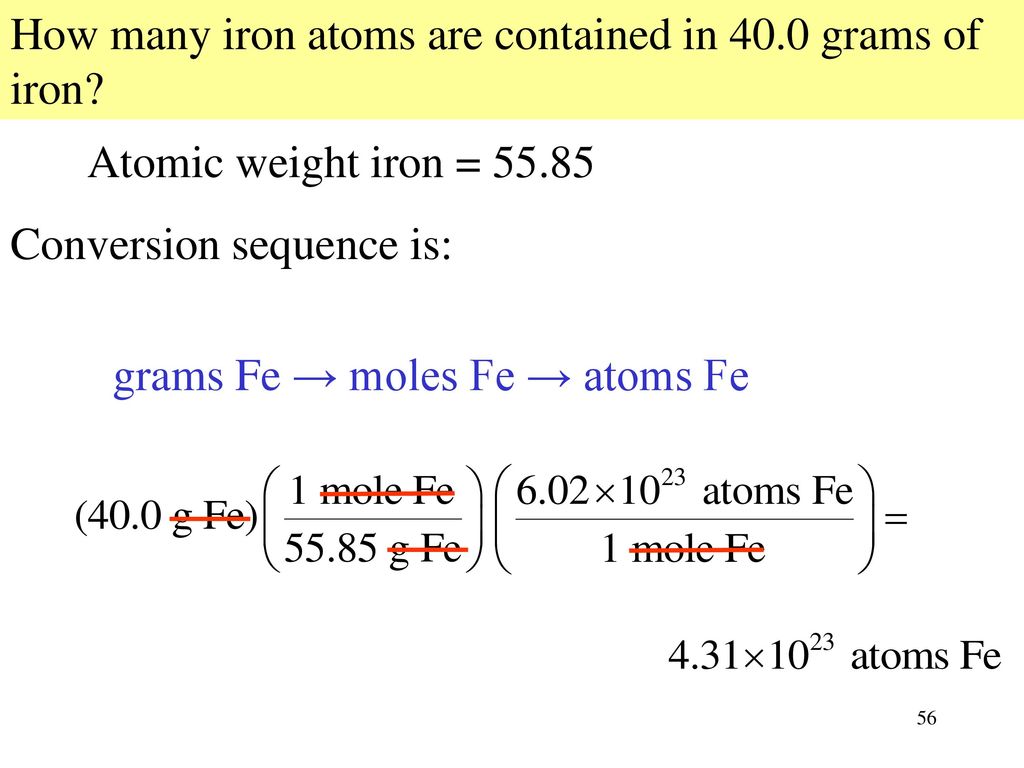
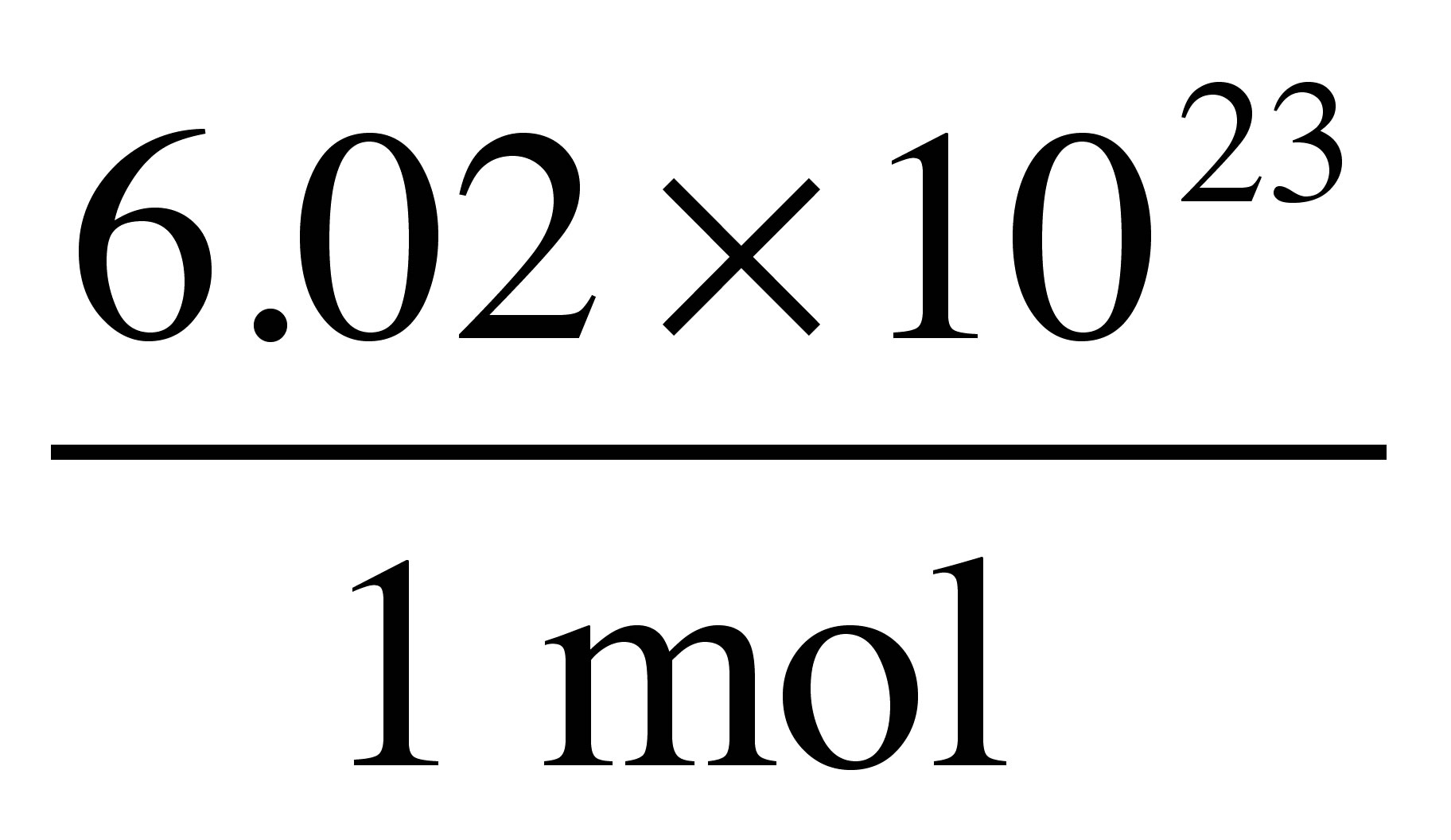Calculate the number of iron atoms in a piece of iron weighing `2.8 g` ( Atomic mass of iron `= 56 u` - YouTube

1. 2 The Mole 3 Molly the Mole 4 The mass of a single atom is too small to measure on a balance. mass of hydrogen atom = x g. - ppt download

7. Calculate the ratio of atoms present in 4 g of magnesium and 4 g of iron. (Atomic masses: Mg = 24, Fe - 56) form ICHCL